Society
Quality Management
Policy
Policy (our fundamental view)
We have established our Quality Policy in order to “provide the highest quality products and services” as stated in the Code of Conduct of the NOF Group Corporate Philosophy System, and strive to carry out sound quality management.
Quality Policy
The NOF Group provides the highest quality products and services that are based on our considerations of achieving harmony with society and customer satisfaction through our unrelenting quality management.
1. Harmony with society
We shall comply with laws, regulations, and rules, respect the environment, and produce safe and secure products using manufacturing processes that themselves are safe and secure.
2. Customer satisfaction practices
We shall listen to our customers’ requirements and provide products that meet expectations and useful information.
3. Unrelenting quality management
We shall maintain stable quality with continuous improvement and proper process management and enhance the management level through education.
4. Execution of quality assurance
We shall fulfil our commitment to our customers and sincerely respond to inquiries and complaints.
Organizational setup
In April 2019, NOF established the Quality Management Committee to supervise quality management and continue to secure the trust of society. The Committee is chaired by the General Manager of the Corporate Technical Division (concurrently Director and Executive Operating Officer) and has 9 other members (business division managers, related corporate division managers). Furthermore, in April 2023, the Quality Management Department was established in the Corporate Technical Division to maintain and improve the level of quality management across the entire Group by raising awareness of quality management and providing guidance on quality management systems (QMS*) operated by works, plants, and Group companies.
Handling complaints and accidents related to products
In the event of consumer complaints regarding the function or quality of NOF products, or consumer accidents (product liability incidents) caused by, or assumed to have been caused by product defects, we organize a business division task force and respond in accordance with the direction of the RC Committee chair. In fiscal 2024, there were no product liability incidents.
QMS
Development status of QMS
NOF’s works and plants have acquired the most appropriate QMS certification for their business from competent external bodies. The Functional Materials and Metal Coatings businesses are certified under ISO 9001, the Explosives & Propulsion business is certified under JIS Q 9100, and the Functional Foods business is certified under FSSC 22000 (Food Safety Management System). The Life Science business utilizes ICH-Q7 (Active Pharmaceutical Ingredients: GMP Guidelines). Fifteen Group companies have acquired certification by competent external bodies, including ISO 9001 certification.
Rate of acquisition of QMS certification from competent external bodies (production volume basis)
- FY2024[Covered organizations:Domestic Group]:98%
- FY2024[Covered organizations:NOF Group]:97%

NOF
| Works and plants | Quality management systems | Certification number | Latest recertification date | |
|---|---|---|---|---|
| Kawasaki Works | Chidori Plant | ISO 9001 | JP026326 | June 2025 |
| Daishi Plant | FSSC 22000 (Food Safety Management System) |
JMAQA-FC270 | January 2025 | |
| DDS Plant | ICH-Q7 (Active Pharmaceutical Ingredients: GMP Guidelines) |
- | - | |
| Aichi Works | Taketoyo Plant | JISQ 9100 | JQA-AS0183 | July 2022 |
| Kinuura Plant | ISO 9001 | JP022549 | June 2022 | |
| Amagasaki Plant | (Functional Materials Division) |
ISO 9001 | JP022753 | August 2022 |
| (Life Science Division) | ICH-Q7 (Active Pharmaceutical Ingredients: GMP Guidelines) |
- | - | |
| Oita Plant |
ISO 9001 | JP023986 | September 2023 | |
Group companies
| Name | Quality management systems | Certification number | Latest recertification date |
|---|---|---|---|
| Nippon Koki Co., Ltd. | ISO 9001 | JSAQ2282 | September 2023 |
| Nippon Koki Co., Ltd. Shirakawa Plant | JISQ 9100 | JQA-AS029 | September 2023 |
| NiGK Corporation | ISO 9001 | 66885 | March 2023 |
| NOF METAL COATINGS ASIA PACIFIC CO., LTD. |
ISO 9001 | JP023061 | November 2022 |
| Showa Kinzoku Kogyo Co., Ltd. | ISO 9001 | 02479-2011-AQ-KOB-JAB | March 2024 |
| YUKA SANGYO CO., LTD. | ISO 9001 | 19823082 | April 2024 |
| JEUNE BEAUTY CORPORATION, Amagasaki Factory | ISO 22716 (cosmetics materials GMP) |
JP024379 | January 2024 |
| JEUNE BEAUTY CORPORATION, Chita Factory |
ISO 22716 (cosmetics materials GMP) |
JP024609 | March 2024 |
| Nichiyu Techno Co., Ltd. | ISO 9001 | JP024473 | February 2024 |
| NIKKA COATING CO., LTD. | ISO 9001 | 3357 | December 2024 |
| Changshu NOF Chemical Co., Ltd. | ISO 9001 | CN20/21619 | January 2024 |
| PT.NOF MAS CHEMICAL INDUSTRIES | ISO 9001 | ID00/18019 | April 2023 |
| NOF METAL COATINGS NORTH AMERICA INC. |
ISO 9001 | 66561-IS8 | July 2023 |
| NOF METAL COATINGS EUROPE S.A. | ISO 9001 | BR040177 | December 2023 |
| NOF METAL COATINGS EUROPE N.V. | ISO 9001 | 10477422 | November 2022 |
| NOF METAL COATINGS KOREA CO.,LTD. | IATF 16949 | RTS0250 | November 2023 |
| NOF METAL COATINGS SOUTH AMERICA IND. E COM.LTDA. | ISO 9001 | N°1994/2984.9 | December 2023 |
| NOF METAL COATINGS SHANGHAI CO., LTD. |
ISO 9001 | 016SH22Q33019R3S | September 2023 |
Approach to Pharmaceutical-Related Products
NOF currently handles pharmaceutical-related products based on the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (“Pharmaceuticals and Medical Devices Act”), and has obtained the various business licenses listed below. In order to properly carry out these licensed business operations, the Pharmaceutical Management Task Force has been established under the Quality Management Committee to appropriately manage medical supplies.
Licensing status of pharmaceutical-related products
| Category | Works / plants | |
|---|---|---|
| Marketing | Second-class marketing license for pharmaceuticals | NOF |
| Marketing license for quasi- pharmaceutical products | NOF | |
| Sales | Wholesale pharmaceutical sales | NOF |
| Manufacturing | Pharmaceutical manufacturing | Kawasaki Works |
| Aichi Works Taketoyo Plant | ||
| Amagasaki Plant | ||
Basic Policy on Pharmaceutical-Related Products
Based on our corporate philosophy, which states, “the NOF Group is dedicated to contributing to humanity and society as a corporate group that creates new value through the power of chemistry, from the biosphere to outer space,” NOF is developing pharmaceutical-related business that ensures high quality, reliability, and safety for all stakeholders under our governance system that ensures thorough compliance with laws and regulations.
Pharmaceutical management system
Based on the aim of the Act Partially Amending the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (Act No. 63 of 2019, “Amended Pharmaceuticals and Medical Devices Act”), Officers with responsibility for pharmaceutical-related operations are clearly stated in the Pharmaceutical Management System. In addition, in accordance with the provisions of the same Act, for marketing (second-class marketing license for pharmaceuticals, marketing license for quasi-pharmaceutical products), a General Marketing Manager, Quality Assurance Manager, and Safety Management Manager have been appointed.
As a specialized task force of the Quality Management Committee, a deliberative body, NOF established the Pharmaceutical Management Task Force to oversee the pharmaceutical-related business of the entire Company and provide integrated management of manufacturing, marketing, and wholesale of pharmaceuticals. The Pharmaceutical Management Task Force inspects the status of compliance with GQP, GVP, and GMP ministerial ordinances and operates so as to ensure that the General Marketing Manager, manufacturing managers, and others provide appropriate opinions to responsible Officers regarding issues and problems related to legal compliance.
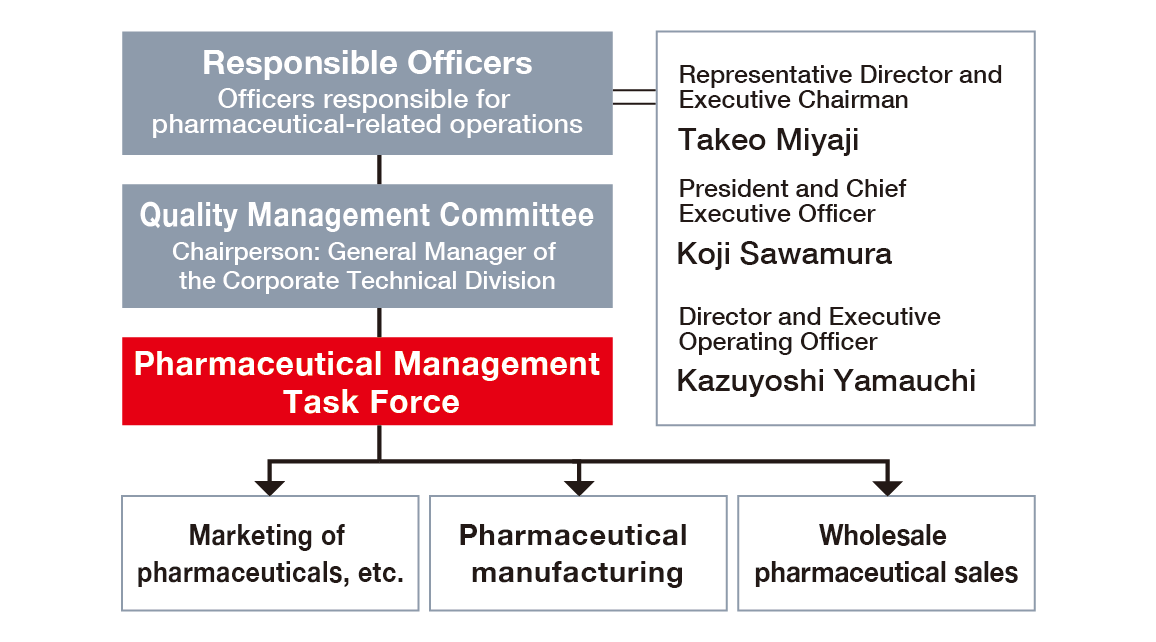
Pharmaceutical management system diagram
Education and training
At NOF, all persons involved in pharmaceuticals take the required training every year.
Responsible Officers:Take the pharmaceuticals-related training (once per year)
People involved in pharmaceutical-related operations:Take training based on GQP, GVP, and GMP ministerial ordinances as needed
After the training, a record is made and stored for use in the next fiscal year’s training plan to ensure continued acquisition of the necessary knowledge.
Risks and Opportunities
Risks and opportunities in quality assurance
- Quality targets:Zero serious complaints or inappropriate incidents
- Key issue:Ensuring proper quality management at the NOF Group
| Major risk | Risk description | Opportunities | Major activities |
|---|---|---|---|
| Quality fraud |
|
|
In 2023, we established a new code of conduct and issued a message from the President to implement specific actions in accordance with the corporate philosophy, which defines the mission and ideal state of the NOF Group, and the three values that are prioritized in implementing the corporate philosophy: Challenges, Fairness, and Harmony. [Thoroughly ensuring proper quality management]
|
| Quality defects and product problems |
|
|



