|
 |

 |
NOF Corporation started the sales of "LIPIDURE®-NR" and "LIPIDURE®-NA",
which have new functions and concepts. The main ingredients of these new products are phosphatide
polymers (LIPIDURE®-S (Fig.1)). LIPIDURE®-NR is a solution of polymers
in multivalent alcohol. LIPIDURE®-NA is dispersion of nano-particles of polymer (Ave.
particle size: 50nm) in water.
|
 |
 |
"LIPIDURE®-NR" and "LIPIDURE®-NA" have unique characteristics. They
form nano scale lamella structures on an applied surface when they dry.
|
 |
 |
Transmission Electron Microscopic observation of a casting film of LIPIDURE®-NA shows
formation of a lamella structure.
|
 |
 |
The new products are effective cosmetic ingredients for skin care and hair care. They restore and
protect the skin and hair healthy.
|
 |
 |
1.LIPIDURE®-NR
|
 |
 |
(Product)
|
 |
 |
5% LIPIDURE®-S solution in multivalent alcohol, which is a mixture of Glycerin and
1,3-Butylene Glycol.
INCI names: Glycerin, Butylene Glycol, and Polyquaternium-61
|
 |
 |
(Characteristics)
|
 |
 |
LIPIDURE®-NR
Encloses and reserves active components stably,
Forms lamella structure on the skin surface and protects the skin, and
Increases the penetration efficiency of active components.
|
 |
 |
(Demonstration of Reserving effect)
|
 |
 |
LIPIDURE®-NR can enclose oily active components such as Vitamins. The enclosed
ingredients were confirmed to remain on the hair surface. Here, the model material used as an active
component was florescent oil. Fluorescence was incorporated in LIPIDURE®-NR.
Observations under a fluorescent microscope of damaged hair that was immersed into the
LIPIDURE®-NR showed fluorescence.
|
 |
 |
| (1) |
Inclusion of the fluorescent probe (active components) into
LIPIDURE®-NR LIPIDURE®-NR dissolves into water by mixing for 30 min
at 50 to 60?C. While stirring water of 50 to 60?C, just add LIPIDURE®-NR to which
fluorescence is included. With this simple method, nano particles are formed. There is no need to
use ultra high-presser homogenizer (ex. microfluidizer). |
|
 |
 |
| (2) |
Microscopic photographs of 1) non-treated damaged hair, 2) fluorescence
dissolving in water from surfactant-treated hair, and 3) inclusion of fluorescence in
LIPIDURE®-NR treated hair. |
|
 |
 |
Only LIPIDURE®-NR treated hair showed strong
fluorescence. This shows that LIPIDURE®-NR with active components remained on the hair
even after the hair was rinsed with water. This is likely because the active component in
LIPIDURE®-NR was included in the newly formed lamella structure on the hair. |
 |
 |
2.LIPIDURE®-NA
|
 |
 |
(Product)
|
 |
 |
1% LIPIDURE®-S water dispersion. LIPIDURE®-S is added to 9.5% Glycerin,
9.5% 1,3-Butylene Glycol and 0.25% PCA Ethyl Cocoylarginate, which is a preservative.
INCI names: Water/Glycerin/Butylene Glycol/Polyquaternium-61/PCA Ethyl Cocoylarginate
|
 |
 |
(Characteristics)
|
 |
 |
LIPIDURE®-NA
Repairs damaged hair by forming lamella structure on the hair,
Makes the hair antistatic, and
Protects the hair from discoloring.
|
 |
 |
(Demonstration of the repair efficacy of damaged hair)
|
 |
 |
We observed the wetting effect under a high power microscope. A drop of water on the surface of a
healthy hair fiber, damaged hair by bleaching, and damaged hair that was treated with hair care
ingredient solutions (active conc. 0.05%)
|
 |
 |
Damaged hair showed decreases in contact angle with water from that of healthy hair. Because the
lipid phase at the outermost surface on the hair fiber was damaged, the hair surface became
hydrophilic. On the other hand, LIPIDURE®-NA treated hair showed high repellency
similar to that of healthy hair. LIPIDURE®-NA restored the hair fiber to be hydrophobic
again by forming a lamella structure on the hair. The damaged hair was restored into healthy hair.
|
 |
 |
3.Safety
|
 |
 |
Safety of LIPIDURE®-NR and LIPIDURE®-NA
|
 |
 |
| Item |
Result |
| LIPIDURE®-NR |
LIPIDURE®-NA |
| Acute oral toxicity test |
>2000mg/kg |
>2000mg/kg |
| Skin primary irritation test |
Non-irritation |
Non-irritation |
| Human patch test |
negative |
negative |
Table 1.
|
 |
 |
| Item |
Result |
| Acute oral toxicity test |
>2000mg/kg |
| Mutagenicity test |
negative |
| Skin primary irritation test |
Non-irritation |
| Continuous skin irritation test |
Non-irritation |
| Ocular irritation testing |
Very slight |
| Skin sensitization testing |
Non-sensitization |
| Human patch test |
negative |
Table 2.
|
 |
|


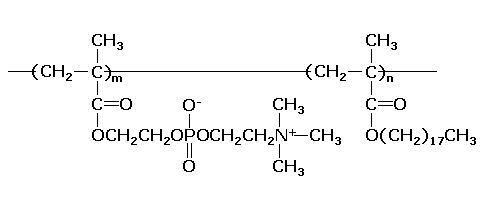
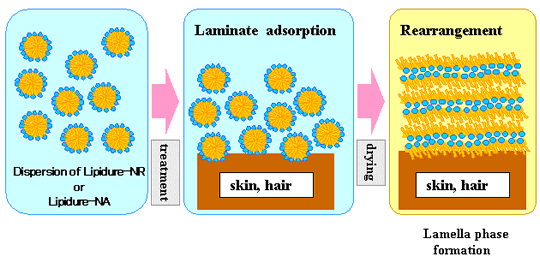
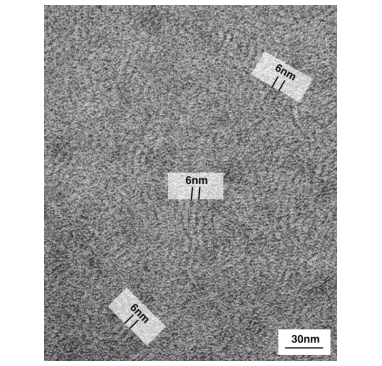
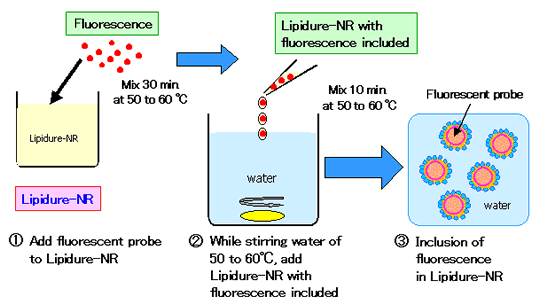
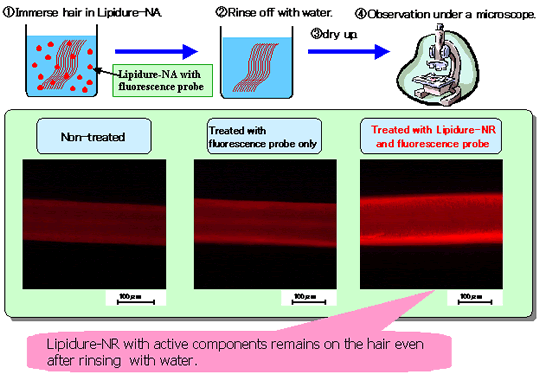
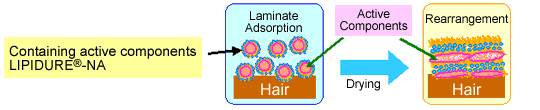 Figure 5.
Figure 5.
