Product information
- TOP
- Business
- Functional Materials
- Organic peroxides
Organic Peroxides
Organic peroxides may generally be considered as derivatives of hydrogen peroxides(H2O2)in which one or both of the hydrogen atoms are replaced by an organic group. Organic peroxides contain the -O-O- bond within the molecular structure, and the chemical properties of the peroxides originate from this bond.
Organic peroxides can be decomposed thermally at relatively low temperatures. They also easily produce free radicals by reaction with reducing substances. The common properties of the free radicals are the addition reaction to an unsaturated double bond and the hydrogen abstraction reaction. Based on the addition reaction, peroxides are widely used as polymerization initiators for various synthetic resins (LDPE, PVC, PS, ABS, PMMA, etc.) or as curing agents for unsaturated polyester resins, vinyl ester resins, etc. Based on the abstraction reaction, they are used as crosslinking agents for a variety of synthetic rubbers and synthetic resins, fluidity modifiers for polypropylene, and agents for grafting maleic anhydride to polyolefins.
- 【Polymerization initiators】
-
Polymerization initiators
Polymerization initiators Vinyl Chloride Ethylene (high Press.) Styrene AS ABS MBS Acrylic Ester Synthetic Rubber Vinyl Acetate Acrylonitrile Tetrafloroethylene MMA Vinyliden Chloride Ketone Peroxide PERHEXA® H PERMEK® G PERMEK® N,H,S,F Peroxy Ketal PERHEXA® C ○ ◎ ◎ ○ ○ PERHEXA® V ○ ○ ○ ○ ○ PERHEXA® 22 ○ ◎ ◎ ○ ○ PERTETRA® A ○ ◎ ◎ ○ ○ Hydroperoxide PERBUTYL® H ◎ ○ ◎ ◎ ○ ◎ ○ ○ PERCUMYL® H ○ ○ ◎ ◎ ◎ ○ ○ PERCUMYL® P ◎ ◎ PERMENTA® H ◎ ◎ PEROCTA® H ◎ ○ ◎ ◎ ○ ○ ◎ Dialkylperoxide PERBUTYL® C ◎ ◎ PERBUTYL® D ◎ ◎ ○ ○ ○ PERBUTYL® P
PERCUMYL® D ◎ PERHEXA® 25B ○ PERHEXYNE 25B Diacylperoxide NYPER® BMT ○ ◎ ○ ◎ ◎ ○ ◎ ○ NYPER® BW,FF,BO, ○ ◎ ○ ◎ ◎ ○ ◎ ○ PEROYL® IB ◎ ○ ○ PEROYL® L ◎ ○ ◎ ◎ ◎ ○ ○ ○ ◎ PEROYL® SA ○ ○ ○ ◎ PEROYL® 355 ◎ ○ ○ ○ ○ ○ ○ ◎ ○ Peroxydicarbonate PEROYL® IPP ○ ○ ◎ ○ ◎ PEROYL® NPP ○ ○ ◎ ○ ◎ PEROYL® OPP ◎ ◎ ○ Peroxyester PERBUTYL® A ◎ ○ ○ ○ ○ PERBUTYL® E ◎ ◎ ◎ ○ ○ ○ PERBUTYL® I ◎ ◎ ◎ ○ ○ ○ PERBUTYL® ND ◎ ◎ ○ ○ ◎ ○ PERBUTYL® O ◎ ◎ ◎ ◎ ◎ ◎ ○ PERBUTYL® PV ◎ ◎ ○ ◎ ◎ ◎ PERBUTYL® Z ◎ ◎ ◎ ○ ◎ ○ PERBUTYL® 355 ○ ○ ○ ○ ○ ○ PERCUMYL® ND ◎ ○ ○ ○ ○ ○ PERHEXA® 25O ○ ◎ ◎ ◎ ○ PERHEXA® 25Z ○ ◎ ○ ○ PERHEXYL® ND ◎ ○ ○ ○ ○ ○ PERHEXYL® PV ◎ ◎ ○ ○ ◎ PEROCTA® ND ◎ ○ ○ ○ ○ ○ PEROCTA® O ○ ◎ ◎ ◎ ○ ◎ ○
- 【Crosslinking agents】
-
Resin Concrete Synthetic Marble Hand-Lay-up Spray-up RI Vinyl Ester Resin FW Pultrusion Continuous Corrugated Sheet Matched Metal Die Acrylic Resin SMC BMC DAP Resin Ketone Peroxide PERHEXA® H ○ ○ ○ ○ ○ ○ ○ ○ ○ ○ PERMEK® G ○ ○ ○ ◎ ○ ○ ○ ○ ○ ○ PERMEK® N,H,S,F ◎ ◎ ◎ ◎ ◎ ◎ ◎ ○ ◎ ○ Peroxy Ketal PERHEXA® C ○ ○ ○ ○ ○ ◎ ◎ ○ PERHEXA® V ○ ○ ○ ○ PERHEXA® 22 ○ ○ ○ ○ ○ ○ ○ PERTETRA® A Hydroperoxide PERBUTYL® H ○ ○ ○ ○ ○ PERCUMYL® H ◎ ◎ ◎ ○ ○ ◎ ○ ◎ PERCUMYL® P ○ ○ ○ ○ ○ PERMENTA® H ○ ○ ○ ○ ○ PEROCTA® H ○ ○ ○ ○ ○ Dialkylperoxide PERBUTYL® C ○ ○ ○ PERBUTYL® D ○ ○ ◎ PERBUTYL® P
○ ○ ○ PERCUMYL® D ◎ ◎ ◎ PERHEXA® 25B ○ ○ ○ PERHEXYNE 25B ○ ○ ○ Diacylperoxide NYPER® BMT ◎ ◎ ○ ○ ◎ ◎ ◎ ◎ ◎ ◎ ◎ ○ ○ ◎ NYPER® BW,FF,BO, ◎ ◎ ○ ○ ○ ◎ ◎ ◎ ◎ ◎ ○ ○ ◎ PEROYL® IB ○ ○ ○ PEROYL® L ○ ○ ○ ○ ○ PEROYL® SA ○ ○ ○ ○ ○ PEROYL® 355 ○ ○ ○ ○ ○ Peroxydicarbonate PEROYL® IPP ○ ○ ○ PEROYL® NPP PEROYL® OPP Peroxyester PERBUTYL® A PERBUTYL® E ○ ○ ○ ○ ◎ ◎ ◎ ○ PERBUTYL® I ○ ○ ○ ○ ◎ ◎ ◎ ○ PERBUTYL® ND PERBUTYL® O ○ ◎ ○ ◎ ○ ◎ ○ ◎ ◎ ○ PERBUTYL® PV ○ ○ ○ ○ ○ ○ ○ PERBUTYL® Z ○ ○ ○ ○ ◎ ◎ ◎ ◎ PERBUTYL® 355 ○ ○ ○ ○ ◎ ○ ○ ○ PERCUMYL® ND PERHEXA® 25O ○ ○ ○ ○ ○ ○ ○ ○ PERHEXA® 25Z ○ ○ ○ ○ ○ ◎ ◎ ◎ PERHEXYL® ND PERHEXYL® PV ○ ○ ○ ○ ○ ○ ○ PEROCTA® ND PEROCTA® O ◎ ○ ◎ ○ ◎ ○ ◎ ○ ○
- 【Curing agents & Polymer modifier】
-
Crosslinking Agents Polymer
ModifierPolyethylene EVA EPT Synthetic Rubber Natural Rubber Silicone Rubber PP Melt Flow Modification Grafting Agents Ketone Peroxide PERHEXA® H PERMEK® G PERMEK® N,H,S,F Peroxy Ketal PERHEXA® C ◎ ◎ ◎ ◎ ○ ○ ○ ○ PERHEXA® V ◎ ◎ ◎ ◎ ○ ○ ○ ○ PERHEXA® 22 ○ ○ PERTETRA® A Hydroperoxide PERBUTYL® H PERCUMYL® H PERCUMYL® P PERMENTA® H PEROCTA® H Dialkylperoxide PERBUTYL® C ◎ ◎ ◎ ◎ ◎ ◎ ◎ ◎ PERBUTYL® D ◎ ◎ ◎ ◎ ◎ ◎ ◎ ◎ PERBUTYL® P
◎ ◎ ◎ ◎ ◎ ◎ ◎ ◎ PERCUMYL® D ◎ ◎ ◎ ◎ ◎ ◎ ◎ ◎ PERHEXA® 25B ◎ ◎ ◎ ◎ ◎ ◎ ◎ ◎ PERHEXYNE 25B ◎ ◎ ◎ ◎ ◎ ◎ ◎ ◎ Diacylperoxide NYPER® BMT ○ ○ ○ NYPER® BW,FF,BO, ◎ ○ ○ PEROYL® IB PEROYL® L PEROYL® SA PEROYL® 355 Peroxydicarbonate PEROYL® IPP PEROYL® NPP PEROYL® OPP Peroxyester PERBUTYL® A ○ PERBUTYL® E ○ ○ PERBUTYL® I ○ ○ PERBUTYL® ND PERBUTYL® O ○ PERBUTYL® PV PERBUTYL® Z ○ ○ ○ ○ ○ ○ ◎ ○ PERBUTYL® 355 ○ PERCUMYL® ND PERHEXA® 25O ○ PERHEXA® 25Z ◎ ◎ ◎ ○ ○ ○ ◎ ○ PERHEXYL® ND PERHEXYL® PV PEROCTA® ND PEROCTA® O ○
- 【General properties of organic peroxides】
-
General properties of organic peroxides
Organic peroxides may generally be considered as derivatives of hydrogen peroxides (H2O2) in which one or both of the hydrogen atoms are replaced by an organic group. Organic peroxides contain the -O-O- bond within the molecular structure, and the chemical properties of the peroxides originate from this bond.
Organic peroxides may be classified by chemical structure as follows:
Type Structure Hydroperoxides 
Dialkylperoxides 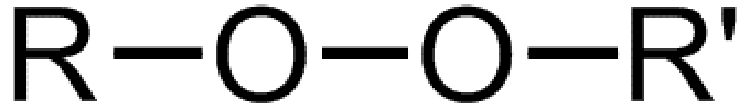
Peroxyesters 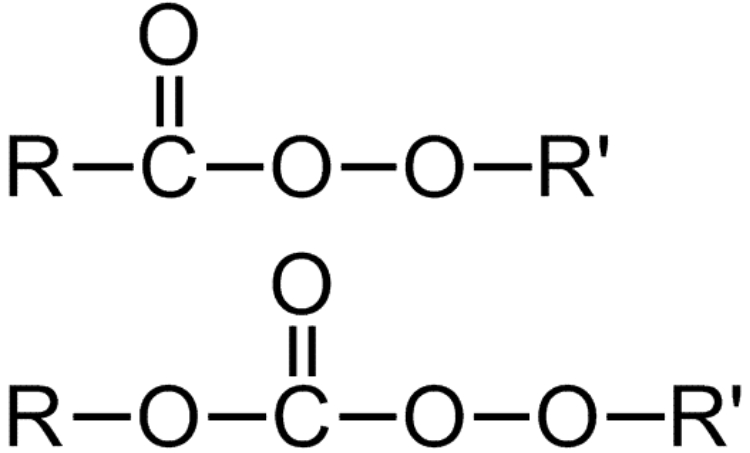
Diacylperoxides 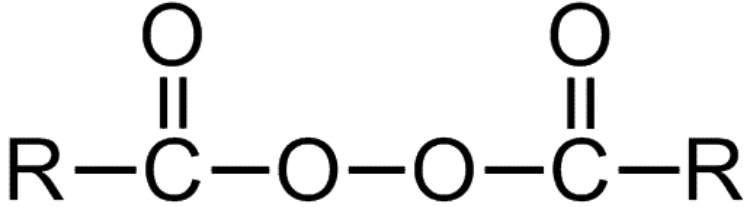
Peroxydicarbonates 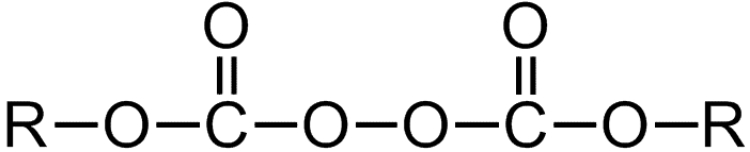
Peroxy ketals 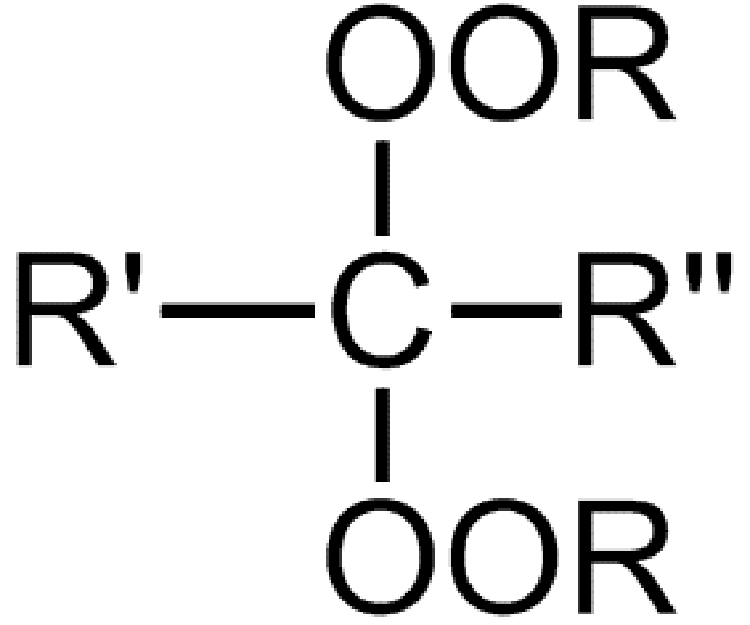
Ketone peroxides 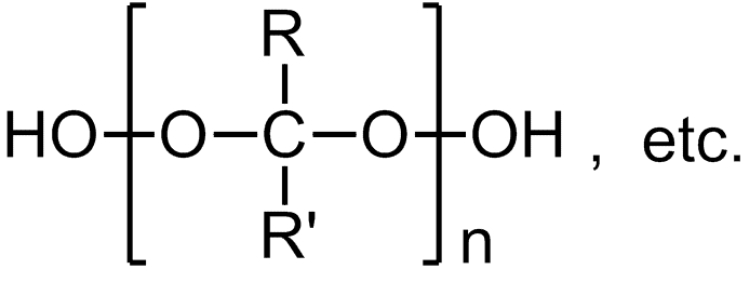
There are various types of organic peroxides, e.g. refrigerated organic peroxides undergoing self-accelerating thermal decomposition below room temperature, stable organic peroxides which do not generate appreciable amounts of free radicals without being heated to high temperatures, and/or some peroxides which generate radicals reacting with an accelerator.
- 【Selection of organic peroxides】
-
Selection of organic peroxides
Organic peroxides have various structures and decompose at different temperatures to generate free radicals.
Accordingly, to choose the most suitable organic peroxides for a purpose, it is necessary to know the properties of a peroxide.
Data of characteristic values such as active oxygen content, half-life, and activation energy of peroxides are important information.
Moreover, organic peroxides are supplied as both the pure form and as mixtures with a diluent to increase stability. In the latter case, the effect of the diluent on conditions of polymerization must be considered.
The diluent can be specified by the customer, unless it influences the stability of the organic peroxide.Active oxygen content
Active oxygen content represents not only the amount of free radicals produced from the peroxide but also the concentration or the purity of the products, i.e., the theoretical active oxygen content of 100% pure peroxide is given (Table 1) by the percentage of atomic weight of active oxygen to the molecular weight of the peroxides.

Half-life and activation energy
Half-life is a convenient index which represents the decomposition rate of organic peroxides from the initial active oxygen content of the peroxide to half of that value by decomposition at a specific temperature.
Half-life is measured using a solution of 0.1mol/l (occasionally 0.05mol/l) of peroxide with a solvent relatively inert to radicals, e.g. benzene, under nitrogen sealed in a glass ampoule, and immersed in a constant temperature bath set to the temperature required.
Generally, decomposition of organic peroxide can be treated approximately as a first order reaction as follows: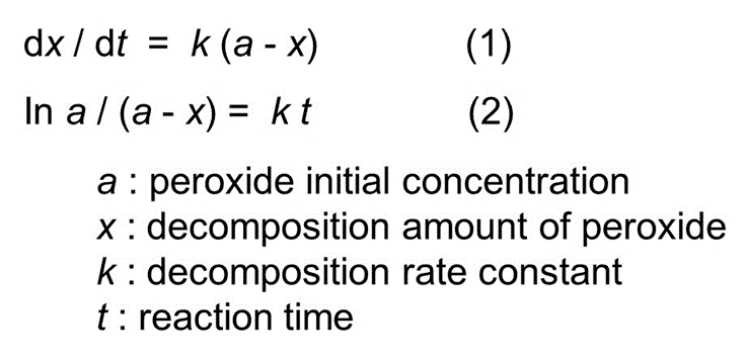
Half-life (t1/2) is the time required to reduce (a) to (a/2) by decomposition, so equation (3) can be obtained by substituting (a/2) for (x) in equation (2).

Therefore, organic peroxide decomposition at a specific temperature has a linear relationship between time (t) and [ln a/(a-x)], so (k) can be obtained from the slope of the straight line. Then, half-life (t1/2) can be obtained at the specific temperature from equation (3).
The rate constant (k) is given as follows: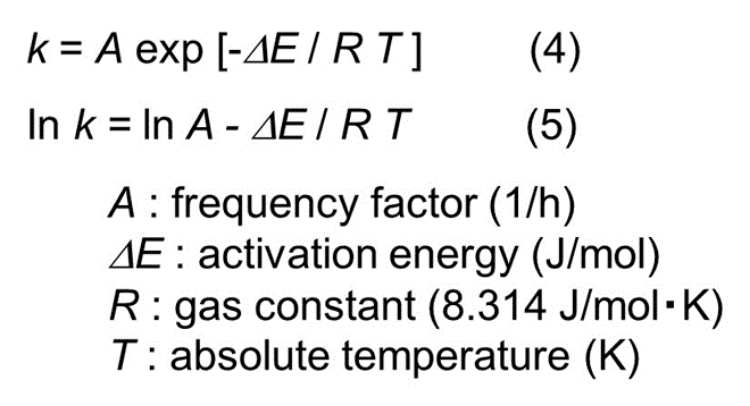
Measurement of (k) at several temperatures provides the relationship between (lnk) and (1/T), so activation energy can be obtained from the slope of the straight line.
The straight line relationship of (lnt1/2) to (1/T) can be used to find the half-life of organic peroxide at any temperature, or the decomposition temperature required for a particular half-life.Decomposition products
During the polymerization of monomer and crosslinking of polymers, decomposition products of organic peroxides sometimes cause contamination, and have a bad influence on the physical properties of the polymer. Therefore, when selecting the organic peroxide, it is necessary to consider what decomposition products are generated.
- 【Safety of organic peroxides】
-
Safety of organic peroxides
In general, organic peroxides are unstable to heat, and some types of organic peroxides are very sensitive to friction and shock, and decompose explosively. On the other hand, other types can be handled in a similar way to solvents.
Accordingly, handling of organic peroxides requires adequate knowledge of the characteristics of each organic peroxide. Therefore, it is important to handle organic peroxides by appropriate procedures for safe and efficient use. Adequate assessment techniques for the characteristics of organic peroxides are required, and various testing methods have been developed for this purpose. Based on assessment tests, organic peroxides can be classified into various grades of hazard. The NOF group also has basic data on handling organic peroxides, obtained from various tests conducted at NOF.
- 【Classification by hazard】
-
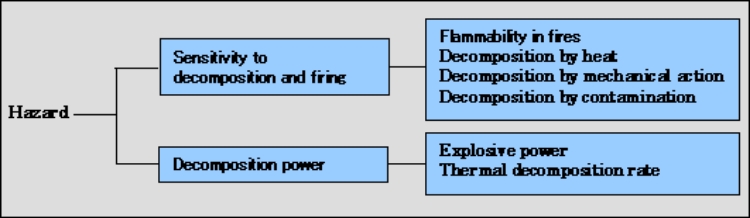
Classification by hazard
The table below shows the classification by hazard of organic peroxides. Various adequate testing methods are used to assess organic peroxides for this classification at NOF. The main testing methods are briefly described below.
Classification by hazard
- 【Various testing methods】
-
Various testing methods
1. Tests on sensitivity to decomposition and firing
Flash point test
(Tag type, Setaflash type, and Cleveland type)
The flash point is defined as the temperature at which ignition of the test material is observed by an open flame over the test material heated to a certain temperature.Rapid heat test
The test material (1g) is put in a test tube, and heated using an electric heating plate at an increasing rate of 4°C/min. to observe the temperature at which the test material starts decomposing and decomposition status.
SADT (BAM method)
[Self-Accelerating Decomposition Temperature]
The test material (400ml) is put in a dewar vessel, which is contained in a chamber in which air at a certain temperature is circulating. This process is carried out to investigate whether the test material decomposes, generates heat (to a temperature increase of over 6°C) under certain temperature conditions, and the rate of decomposition.
A United States SADT test is conducted for the same purpose. This test uses a packaged organic peroxide product available in the market instead of tests using a small amount of organic peroxide in the dewar vessel. However, this method has the disadvantage of difficulty in use from safety and environmental pollution respects.Ignition temperature (ASTM E659 method)
A 500ml flask is placed in a electric furnace with the temperature controlled adequately, and the test material (0.15ml) is put in the flask. In this method, the lowest temperature that induces ignition is measured to decide the ignition temperature (auto-ignition temperature).
Organic peroxides undergo thermal decomposition, and almost all organic peroxides show rapid decomposition accompanied by white smoke before ignition.
Falling weight sensitivity
Testing is carried out using a falling weight sensitivity tester which is used for explosives. The test material (0.1g) is placed on an anvil, and a roller is placed on the test material. A 5kg iron hammer falls on the top of the steel rod, and ignition or explosion of the test material is tested to observe the highest position of the iron hammer not causing ignition or explosion in ten drop tests. This method shows the sensitivity of organic peroxide against impact by a weight as well as the sensitivity to friction.
Friction sensitivity (BAM type)
The tester is equipped with a friction rod and plate made of porcelain. A small amount of the test material is placed between the rod and plate.
Friction motion is caused under a load, and the sensitivity of the organic peroxide is studied from the relationship between load and explosion.2. Decomposition power
Ballistic mortor test
Testing is carried out using a steel block and the No.6 blasting cap in a rigid enclosure. The blasting cap explodes (or decomposes) 10g of the sample. The deflection angle of the weight is compared with a reference of TNT as 100%. This indicates the explosive power of the sample.

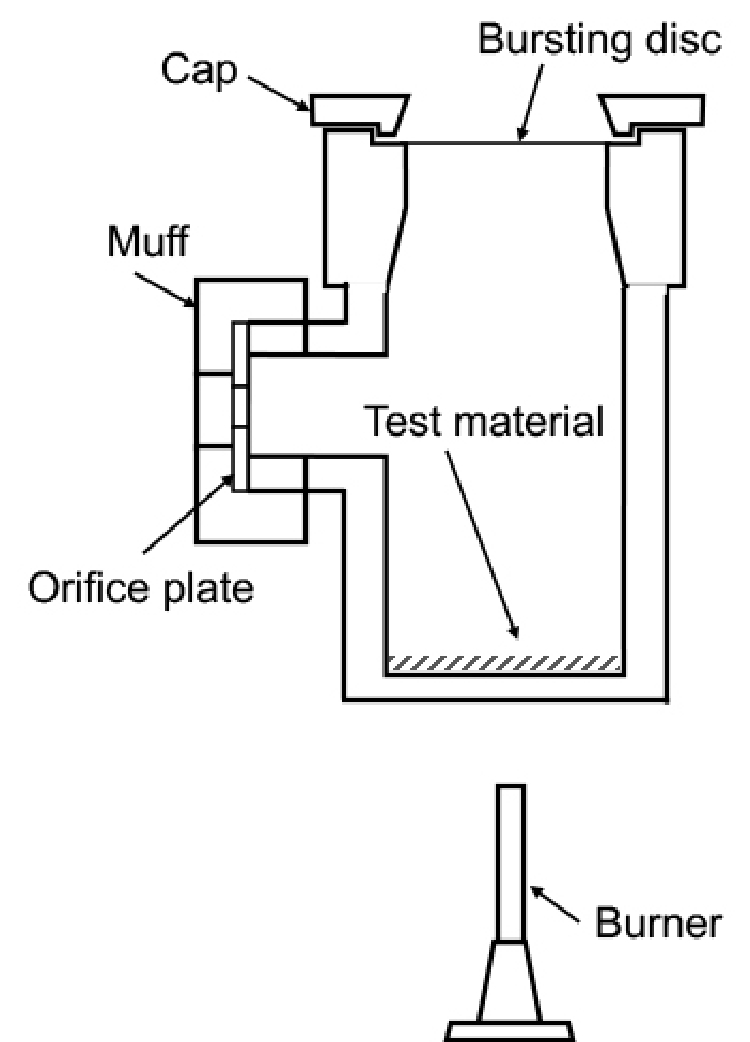
Pressure vessel test
The pressure vessel made of stainless steel is equipped with a bursting aluminum disc with a pressure resistance of 10kg/cm2 and a port for installing a round plate with an orifice of the selected diameter at the side of the vessel. The vessel has an inner capacity of about 235cm3, in which 5g of the test material is placed for thermal decomposition under certain conditions. The violence of decomposition power is measured from the orifice with the minimum diameter required to maintain the inner pressure at 10kg/cm2


DSC
A stainless steel, closed-type vessel is used for tests. About 1mg of the test material is put inside the testing vessel, and is heated up at 10°C/min. The temperature at which decomposition starts (Tb: Temperature at intersectional point) and the heat of decomposition are measured.

-
- 【Handling of organic peroxides】
-
Handling of organic peroxides
Organic peroxides are marketed under conditions with adequate care to avoid danger as described earlier but users must treat the products carefully. The handling instructions described below are principles to handle organic peroxides for safe use of the product.
However, observance of the laws concerning organic peroxides is required, and the directions of the competent authorities must be followed. Please contact the NOF group about any question arising from the contents of this book.Precautions in handling organic peroxides
(1) Thorough care is required to prevent contamination of organic peroxides during handling because contamination may promote decomposition of organic peroxides. When organic peroxides are subdivided to small volumes for use, glass, stainless steel (SUS304 or SUS316), or polyethylene containers must be used. Containers made of steel, copper alloys, lead, rubber, etc. are not useful. Moreover, the subdivided organic peroxide must not be returned to the original container. Containers and vessels containing subdivided organic peroxide must be clearly labeled.
(2) Organic peroxides must not be directly mixed with compounds containing iron, cobalt, manganese, etc., because these compounds cause redox reaction with organic peroxides, and promote the decomposition of organic peroxides. Direct mixing of organic peroxides with amine compounds must also be avoided. When these compounds are used, organic peroxides and these compounds must be separately diluted with resin monomer. After dilution, either may be mixed with the other.
(3) When organic peroxides are mixed with chemicals except for those described in (2), testing is required using a small volume to confirm safety. NOF is ready to give advice to users in advance.
(4) Use of rubber gloves and goggles is advised to avoid contact with organic peroxides which may cause damage the human skin and eyes.
(5) Empty organic peroxide containers must immediately be washed with water, and the cleansed container must be safely kept uncapped in a storehouse to shield the container from sunlight until disposal.
(6) Organic peroxides are very sensitive to fire and heat, and undergo ignition and decomposition, sometimes explosion, so must be used or handled in a room away from ignition source such as electric sparking and high temperatures and heat from radiators, boilers, and other heat sources.
(7) Equipment materials in contact with organic peroxides must be selected from stainless steel, glass lining, glass, and polyethylene. Gaskets made of chemical-resistant materials such as Teflon (polytetrafluoroethylene) are also allowed.
(8) When organic peroxide is treated in a closed container, a temperature monitoring device, safety valve, and a rupture disc must be provided. Residual organic peroxide in equipment and pipes must be thoroughly removed after ceasing machinery operation.
(9) Some types of organic peroxides are very sensitive to friction and shock, so friction or impact to the organic peroxide during handling must be avoided. Accordingly, ground glass is not suitable for use in containers.
(10) When an organic peroxide spills over the floor, etc., a small amount of spillage may be wiped off with a rag for burning disposal at a safe place. A large spillage requires sawdust, diatomite, vermiculite, or dry sand to absorb organic peroxide for appropriate disposal. When temporarily storing a flammable material absorbing organic peroxide, the material must be saturated with water.
(11) To handle organic peroxides requiring temperature control, the necessary amount of organic peroxide must be subdivided, and used immediately. The remaining organic peroxide must be returned to a temperature-controlled storehouse. Organic peroxide reaching room temperature during the period outside the storehouse must not be returned to the storehouse as it is. Organic peroxide reaching a temperature higher than the required storage temperature must be abandoned, or cooled in a water bath below 5°C for return to the storehouse.
Precautions for storage and storing facilities
(1) The storehouse must be cool and dark inside, and organic peroxide must be sheltered from sunlight and separated from radiators, steam pipes, and other heat sources. Organic peroxide inside the storehouse must be kept away from fire.
(2) Organic peroxide must not be stored together with other chemicals. In particular, acids such as sulfuric acid and nitric acid, amines, and metals promote extremely violent decomposition of organic peroxides. Therefore, organic peroxides must be stored separately from these materials.
(3) Organic peroxides must be stored to ensure that containers do not fall down or drop.
(4) Some liquid organic peroxides gradually decompose into gas at room temperature, so containers for such peroxides are provided with a gas ejector on the lid of the container to release gas pressure inside the container. Therefore, the container must be placed with the lid side up for preventing liquid leakage. When organic peroxide is separated into another container, a gas discharge opening must be provided for the container lid.
(5) Explosion-proof electric appliances and machinery must be used for the storehouse.
(6) A refrigerated storehouse must be provided with a thermometer which can be monitored from outside the storehouse.
(7) Refrigerated organic peroxide must be stored at the temperatures recommended in this book.
(8) When a storage facility for organic peroxides is installed, legal regulations are provided for facilities, i.e., the structure and materials used for facilities as well as siting of a facility are regulated. Equipment such as an automatic charging device are required to be safe in use.
Precautions for safe transportation
(1) Containers of liquid organic peroxide must be handled and transported with the top side indicated. Laying a container on its side or upside down is not permitted for safety assurance.
(2) During transportation, containers must be sheltered from direct sunlight with covering materials.
(3) Careful handling and transportation are required for organic peroxides, to avoid friction and vibration, or dropping and falling.
(4) Loading and unloading work for organic peroxides must be carried out at a place without fire. Smoking is prohibited during handling work.
(5) The temperature required for organic peroxide transportation must be maintained.
First aid
(1) When organic peroxide contacts the skin
Repeated cleansing with soap will be sufficient. However, if itching and pain are felt after cleansing, apply an adrenocortical hormone ointment to the skin. For detailed treatment, consult a doctor.(2) When organic peroxide enters the eyes
Eyes must be washed with flowing water for 20 to 30 minutes without any delay, and medical treatment from a physician is necessary. (For reference, when hydroperoxides and methylethylketone peroxides enter the eyes, 5% ascorbic acid sodium solution and 3% sodium bicarbonate solution are useful for cleansing)(3) When organic peroxide is swallowed
The oral toxicity of organic peroxides is not exactly known. When someone has swallowed organic peroxide, it is advisable to induce vomiting after giving a large amount of milk (in the case of hydroperoxides and methylethylketone peroxides, 5% ascorbic acid sodium solution is useful) and immediately seek treatment from a physician. If the patient is unconscious, convulsed, or has a convulsive fit, vomiting should not be induced. Lay the patient on the side, maintain the mouth open for breathing and the head lower than the body, and seek immediate admission to a medical facility.Fire fighting
If a fire involving organic peroxide starts, or there is a fear of igniting organic peroxide, the fire extinguishing method varies according to the surrounding environment at the fire place.
•In case of very small fire
A foam fire extinguisher can be used. After extinguishing the fire, keep pouring water because the residual heat causes decomposition of organic peroxide generating white smoke. If water is poured on the fire at the outbreak of the fire, the fire cannot be extinguished.•In case of fire
As explosion can be anticipated in a fire, fire expansion must be prevented by pouring water over the surrounding area, and fire-fighting personnel should keep at a safe distance. Fire extinguishing agents: water jet, water spray, foams, and reinforcing liquid.Disposal
Organic peroxides that can not be used due to contamination, the guarantee period has expired, or are no longer required, must be treated with utmost care. Disposal of organic peroxides requires extensive knowledge about the properties of the specific organic peroxide to avoid occurrence of an unexpected accident. Refer to Table 3 for disposal of each organic peroxide.
(1) Burning in incinerator<
A boiler as an incinerator can be used for burning disposal of organic peroxide diluted below 10% concentration (or active oxygen 1%) with inert organic solvent. However, this method is not suitable for solid type organic peroxides.(2) Hydrolysis
Hydrolytic disposal uses a solution consisting of 80 parts water, 20 parts sodium hydroxide, and 0.3 parts surfactant, and the required solution is 10 times the weight of organic peroxide to be treated. Organic peroxide must be poured slowly into the solution. During this process, the solution should be stirred to prevent local heat increase. The solution generates heat upon decomposition of organic peroxide but there is no need to cool down the solution. Hydrolytic decomposition proceeds very slowly so stirring for 12 to 24 hours is required, and then organic peroxide is neutralized. After neutralization, separated organic substances can be recovered for burning, and/or biodegradable substances can be decomposed by the activated-sludge process.(3) Activated-sludge process
Biodegradable organic peroxides can be decomposed by the activated-sludge process.



