Product information
- TOP
- Business
- Functional Materials
- Products for oral care and topical use
Products for oral care and topical use
Materials for Oral Care
LIPIDURE® has the ability to prevent the adhesion of bacteria that cause dental caries and can protect mucosal cell from the toxicity of periodontal disease bacteria. Furthermore, LIPIDURE® can suppress bitterness.
Suppressing the adhesion of bacteria that cause dental caries (S. mutans) using LIPIDURE®
LIPIDURE® suppresses the adhesion of bacteria that cause dental caries.
[Test procedure]
- Treated hydroxyapatite plate with saliva and washed it with water.
- Dipped the plate into 1 mL of LIPIDURE® and 1 mL of water for 30 seconds or 10 minutes.
- Washed the plate with water again.
- Added precultured S. mutans, which was incubated at 37°C in anaerobic condition, and washed the plate with saline.
- Observed S. mutans that have adhered to the hydroxyapatite plate using scanning electron microscope (SEM) and measured the number of bacteria.
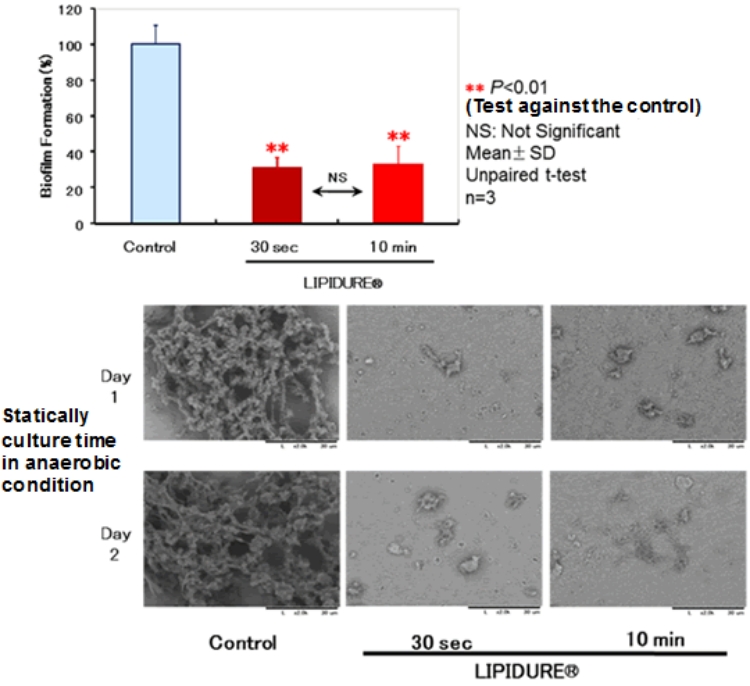
The top figure above shows that applying the LIPIDURE® treatment to a hydroxyapatite plate for more than 30 seconds suppresses the formation of biofilm to about 40%.
Bacteria that cause dental caries produces biofilm for control, but the bottom figure shows that applying the LIPIDURE® treatment for more than 30 seconds reduces the formation of biofilm.
Protecting mucosal cell from the toxicity of periodontal disease bacteria using LIPIDURE®
LIPIDURE® protects the mucosal cell from the toxicity of periodontal disease bacteria.
[Test procedure]
- Cultured gingival epithelial cells.
- Dipped the cell into LIPIDURE® and saline for 30 seconds.
- Washed it with saline again.
- Added the toxin of periodontal disease bacteria (PgLPS).
- Measured the interleukin-8 (IL-8).
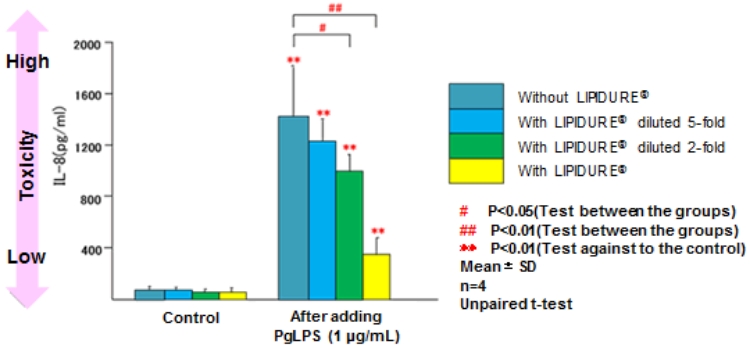
A study conducted by a group led by Prof. Yoichiro Miyake of Tokushima University.
A higher bar indicates higher toxicity. As the concentration of LIPIDURE® changes from blue to yellow, the toxicity decreases.
Suppressing bitterness using LIPIDURE®
[Test procedure]
- Kept the test drug (containing LIPIDURE®) or control drug (not containing LIPIDURE®) in the subject’s mouth, who gargled and spit out the solution.
- Conducted a questionnaire survey about the taste during and after gargling.
* Subjects were blindfolded during the testing to prevent them from distinguishing the sample.
LIPIDURE® has the ability to suppress bitterness.
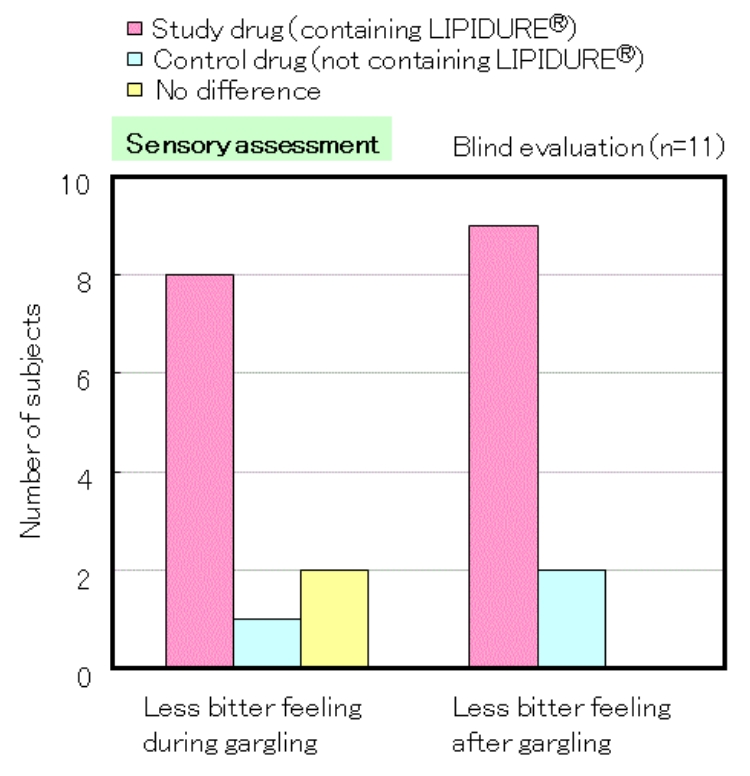
The pink bar indicates the number of subjects who answered that the study drug containing LIPIDURE® is less bitter. The test was performed with a solution containing 0.3% of LIPIDURE®.
Materials for Topical use
LIPIDURE® has excellent skin absorptivity and moisturizing effect, in addition to the ability to protect skin from irritants.
Adsorbing three dimensional cultured skin model using LIPIDURE®
LIPIDURE® has shown that it is able to adsorb three dimensional cultured skin model.
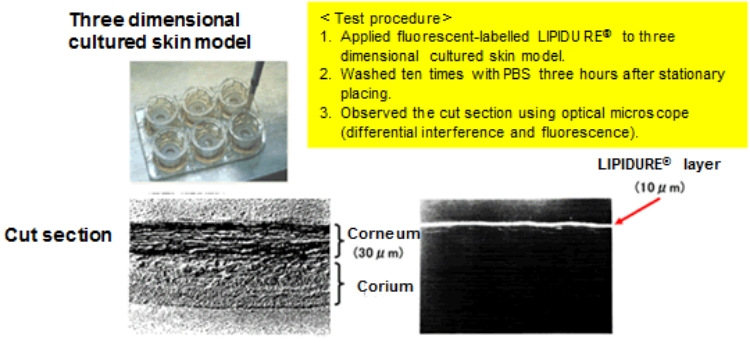
The top figure above shows the LIPIDURE® treatment to three dimensional cultured skin model.
The figure on the bottom left shows the cut section of the three dimensional cultured skin model (bright-field image), while the bottom right shows an image of the differential interference.
In the figure on the bottom right, the LIPIDURE® film on three dimensional cultured skin model turns white and shows that the LIPIDURE® was adsorbed into skin model.
Hygroscopicity and moisture-retaining property of LIPIDURE®
LIPIDURE® becomes less sticky after adsorbing moisture and shows excellent moisture-retaining property.
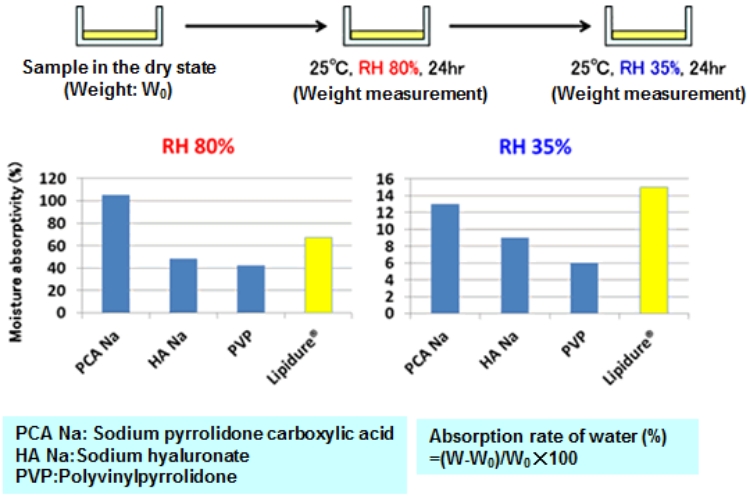
Moisture-retaining property of LIPIDURE® for skin
LIPIDURE® stays on the skin after it is washed with water and shows excellent moisture-retaining property.
[Test procedure]
- Measured the water content in the stratum corneum before applying the sample.
- Measured the water content in the stratum corneum after applying LIPIDURE® and sodium hyaluronate solution (0.2% in terms of pure content for both samples).
- Washed the applied area with water.
- Measured the water content in the stratum corneum of the applied area after one hour.
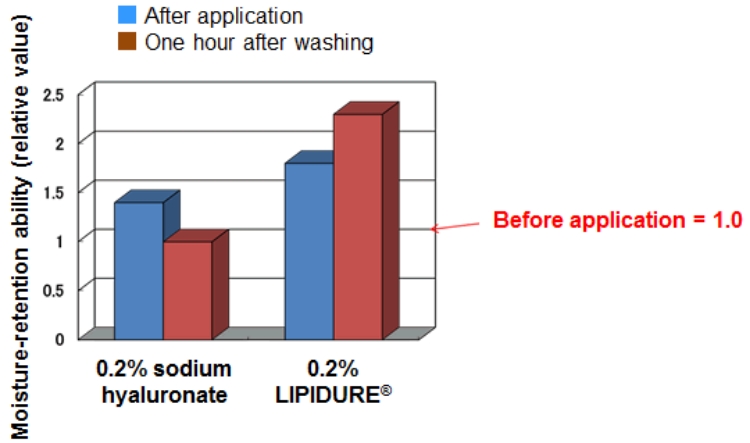
The moisture-retention ability was 1.0 before application and approximately 1.5 after application for both samples. After washing with water, the moisture-retention ability decreased to around 1.0 for sodium hyaluronate solution, while the moisture retention ability increased to around 2.2 for LIPIDURE®.
Alleviating irritation using LIPIDURE®
LIPIDURE® has the ability to alleviate irritation.
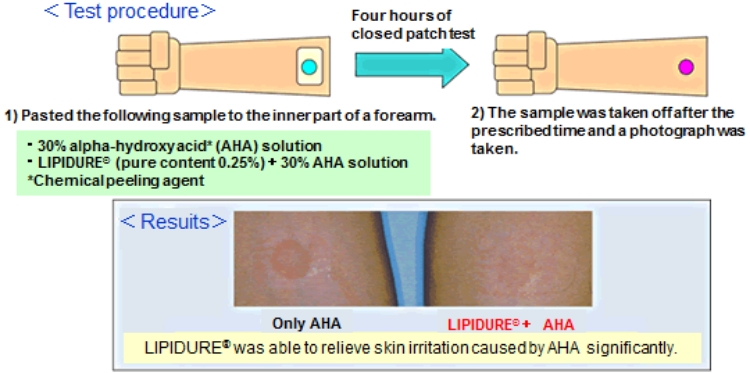
In the bottom figure, significant erythema was identified for AHA only, but erythema disappeared for LIPIDURE®+AHA.
Conference presentation and reference
Hirota, K., Yumoto, H., Miyamoto, K., Yamamoto, N., Murakami, K., Hoshino, Y., Matsuo, T., and Miyake, Y. (2011). J. Dent.Res., 90 (7), 900-905.
Yumoto, H., Hirota, K., Hirao, K., Miyazaki, T., Yamamoto, N., Miyamoto, K., Murakami, K., Fujiwara, N., Matsuo, T., and Miyake, Y. (2015).
J. Biomed. Mater. Res. Part A, 103(2), 555-563.

