SHEKWASHA EXTRACT BG™
Item listed in the “Japanese Standards of Quasi-drug Ingredients”
Extract for beautiful skin from the peel of Shekwasha, a specialty product of Okinawa, Japan
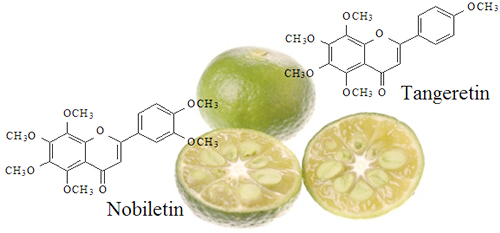
SHEKWASHA EXTRACT BG is an extract from the peel of shekwasha (Citrus depressa Hayata). It is extracted with 1,3-butylene glycol aqueous solution. Shekwasha fruit contains an abundance of nutritional elements such as vitamin C, vitamin B1, and citric acid. Moreover, the peel contains polymethoxyflavonoids (PMFs) such as nobiletin and tangeritin. PMFs are attracting attention for having a variety of pharmacologic effects that enhance health.

1. Features
- Polymethoxyflavonoids (PMFs) demonstrate beauty effects.
PMFs, particularly nobiletin and tangeritin, are effective against lifestyle diseases such as diabetes and high blood pressure. Moreover, PMFs also act to whiten the skin and to suppress the production of matrix metalloproteinases and UV-induced inflammation factor. - Use of raw materials from a clearly identifiable production region
SHEKWASHA EXTRACT BG is made using only shekwasha produced in Okinawa. Fresh shekwasha grown on expansive contracted farms are used as raw materials and processed into this product. - Can be used as an additive in quasi-drugs
SHEKWASHA EXTRACT BG conforms to “Chinpi Extract” as set forth in the Japanese Standard of Quasi-drug Ingredients 2006.
2. Specification
| INCI | Chinese INCI | Content (%) |
|---|---|---|
| CITRUS DEPRESSA PEEL EXTRACT | 扁平橘(CITRUS DEPRESSA)果皮提取物 | 1.4 |
| BUTHYLENE GLYCOL | 丁二醇 | 59.2 |
| WATER | 水 | 39.4 |
Safety tests
- Acute oral toxicity
- Primary skin irritation
- Ocular irritation
- Skin sensitization
- Phototoxicity
- Photosensitization
- Cumulative application
- Mutagenicity (Ames)
- Human patch test
*Implemented all nine safety tests
Product specifications
| Test item | Specification | |
|---|---|---|
| Appearance | Light yellow to yellow-brown liquid, with a faint characteristic odor | |
| Identification (Flavonoids) |
Liquid develops a light to dark red color. (Identification for “Chinpi Extract” in the Japanese Standard of Quasi-drug Ingredients) | |
| pH | 4.0~6.0 | |
| Purity test | Heavy metals | 20 ppm or less |
| Arsenic | 2 ppm or less | |
| Evaporation residue | 0.8~2.0(w/v)% | |
| Residue on ignition | 0.3(w/v)% or less | |
Packaging
1 kg, 2 kg (brown bottle)
3. Functionality
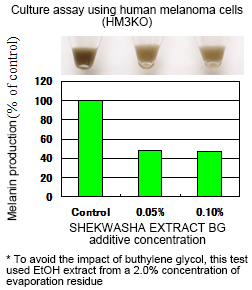
1. Inhibits melanin production
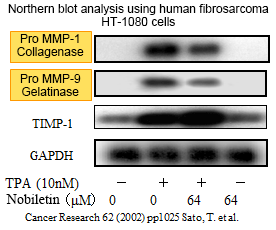
2. Suppresses matrix metalloproteinases (MMPs) production
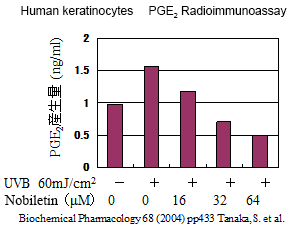
3. Prevents UV-induced skin inflammation

4. Technical data
- SHEKWASHA EXTRACT BG™ Technical data (PDF: 197KB)